-
产品品牌:
REAGEN
-
产品货号:
RNS92048B
-
产品规格:
1/20T
-
产品适用:
适用于新冠病毒检测
-
产地来源:
中国深圳
-
试剂盒组成:
检测卡、拭子、说明书、合格证
-
保质期:
24个月
INTENDEDUSE
The SARS-CoV-2 Antigen IVD Kit SWAB is an in vitro diagnostic rapid test for the qualitative detection of novel coronavirus nucleocapsid antigens in human nasopharyngeal, oropharyngeal swab and nasal swab samples using an immunochromatographic method.
Positive test results suggest the presence of viral antigens, but further clinical evaluation is needed to determine infection status. Bacterial infection or co-infection with other viruses can also produce positive results.
Negative test results do not completely rule out COVID-19 and should be considered in the context of the presence of clinical signs and symptoms.
The test must be carried out by medical professionals.
SUMMARY
COVID-19 is an acute respiratory infectious disease caused by the novel coronavirus SARS-CoV-2. The novel coronavirus SARS-CoV-2 belongs to the β genus, which is an enveloped, non-segmented RNA virus. Currently, the patients infected with the novel coronavirus are the main source of infection, but asymptomatic infected people can also be a source of infection. Based on current epidemiological studies, the incubation period is 1 to 14 days, in most cases 3 to 7 days. The main manifestations are fever, loss of smell and taste, malaise and fatigue, and dry cough. In some cases, there is a runny nose, shortness of breath, muscle pain, and diarrhea.
PRINCIPLE
SARS-CoV-2 Antigen IVD Kit SWAB is an immunochromatographic membrane assay that uses highly sensitive monoclonal antibodies to qualitatively determine the presence of nucleocapsid protein (N-Protein) antigen from SARS-CoV-2 in direct nasopharyngeal swab. When the sample is dropped into the sample well, SARS-CoV-2 antigens in the sample are bound by colloidal gold-labeled monoclonal antibodies against the nucleocapsid protein of SARS-CoV-2. This complex migrates on the membrane via capillary action to the test region (T), where it is captured by the mouse monoclonal anti‑SARS‑CoV‑2. If the SARS-CoV-2 antigens are present in the sample, a colored test line becomes visible in the T line. To serve as a procedural control, a colored line always appears in the control region (C), if the test has performed properly.
REAGENTS
The reagent membrane contains the colloidal-gold conjugated with the monoclonal antibodies against Novel coroinavirus; the reaction membrane contains the secondary antibodies for Novel coroinavirus,and the polyclonal antibodies against the mouse globulin, which are pre- immobilized on the membrane.
DIRECTIONS FOR USE
Allow the test device, specimen, sample extraction buffer to equilibrate to room temperature (15- 30°C) prior to testing. Do not place anything in the mouth including food, drink, gum, tobacco, water and mouth cleaning products
for at least 10 minutes prior to collection of oropharyngeal swab.
Nasopharyngeal Swab Specimen Collection :
1.Remove the swab from the package.
2.Tilt patient's head back about 70°.
3.Insert the swab through the nostril parallel to the palate(not upwards) until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx. (Swab should reach depth equal to distance from nostrils to outer opening of the ear.) Gently rub and roll the swab. Leave swab in place for several seconds to absorb secretions.
4.Slowly remove swab while rotating it. Specimens can be collected from both sides using the same swab, but it is not necessary to collect specimens from both sides if the tip of swab is saturated with fluid from the first collection. If a deviated septum or blockage creates difficulty in obtaining the specimen from one nostril, use the same swab to obtain the specimen from the other nostril.
Nasal Swab Specimen Collection :
1.While gently rotating the swab, insert swab about 2.5 cm(1 inch) into nostril until resistance is met at turbinates.
2.Rotate the swab several times against nasal wall and repeat in other nostril using the same swab.
Oropharyngeal Swab Specimen Collection :
Insert swab into the posterior pharynx and tonsillar areas. Rub swab over both tonsillar pillars and posterior oropharynx and avoid touching the tongue, teeth, and gums.
Testing Procedure :
1.Peel off the aluminum foil seal from a sample extraction tube.
2.Immerse the sampled swab into the sample extraction tube to make the sample extraction buffer completely penetrate the swab, rotate and squeeze the swab 5 times, take out and discard the swab.
3.Insert the tube cap firmly on the sample extraction tube.Gently shake the extraction tube for about 5 seconds to make sure sample mix well with extraction buffer.
4.Transfer 2-3 drops of mixed sample into the test card vertically, start the timer. Read the result at 15 minutes. Don’t interpret the result after 20 minutes.
**Read the result at 15 minutes. Result after 20 mins will not be valid.
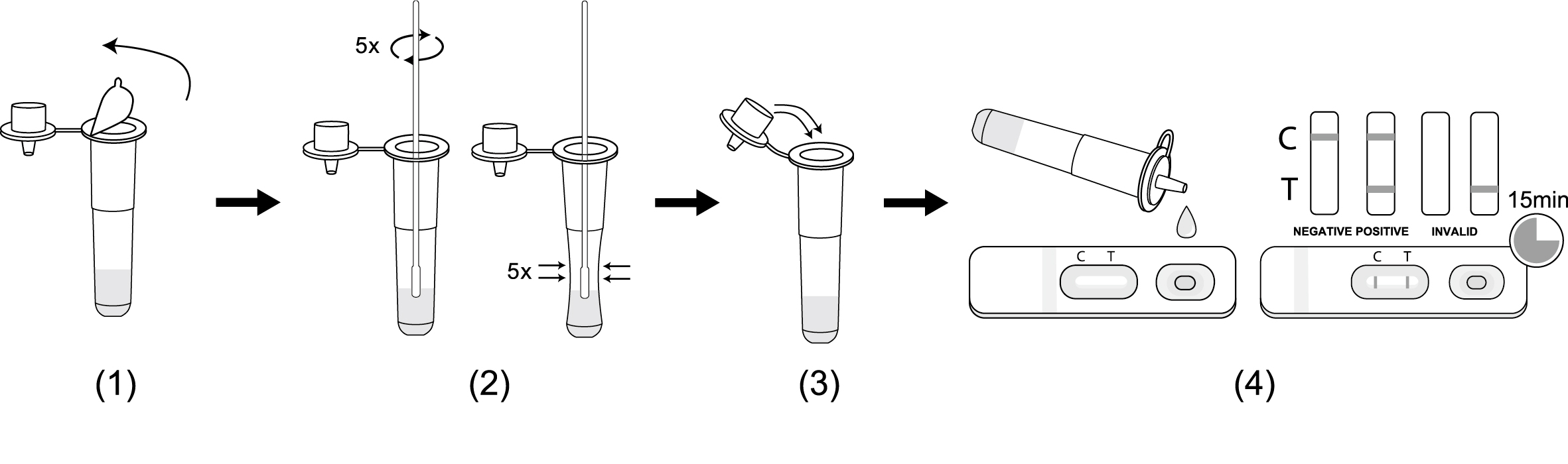
(Please refer to the illustration above)
POSITIVE: Two red lines appear. One red line appears in the control region(C), and one red line in the test region(T). The shade of color may vary, but it should be considered positive whenever there is even a faint line.
NEGATIVE: Only one red line appears in the control region(C), and no line in the test region(T). The negative result indicates that there are no Novel coroinavirus particles in the sample or the number of viral particles is below the detectable range.
INVALID: No red line appears in the control region(C). The test is invalid even if there is a line on test region(T). Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the test procedure and repeat the test using a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
 Reagen-SIMBA-SWAB - DE.pdf
Reagen-SIMBA-SWAB - DE.pdf
 新型冠狀病毒抗原檢測.pdf
新型冠狀病毒抗原檢測.pdf
 08-COVID-19 Antigen Test Performance Evaluation Report(3).pdf
08-COVID-19 Antigen Test Performance Evaluation Report(3).pdf
 10- Summary of the Risk Analysis.pdf
10- Summary of the Risk Analysis.pdf
 04- Product Stability Test Report.pdf
04- Product Stability Test Report.pdf
 19- Clinical Evaluation Report.pdf
19- Clinical Evaluation Report.pdf
 13485.pdf
13485.pdf
 REAGEN SARS-CoV-2 antigen IVD kit SWAB Performance Evaluation Report.pdf
REAGEN SARS-CoV-2 antigen IVD kit SWAB Performance Evaluation Report.pdf



